Bionano Genomics: The Swiss Army knife of cytogenetic testing — and more
Why the genomic revolution needs tools beyond just sequencing — and how Bionano is in a league of its own
Hi friends 👋
Welcome to Health & Wealth — your weekly source of the latest health research and biotech trends. This piece took me a while! It's overdue, but I want to bring you only the highest quality content possible. If you are new, you can join here and share within your network — word-of-mouth is how I grow. Feedback is encouraged and welcome. Happy reading!
Article Highlights
Bionano is flying under the radar in the bubbly genomic space, and you should be aware of it. They develop technology to detect large changes in the genome (structural variations) through a process called optical genome mapping.
This is catching field experts' attention because it's a 3 in 1 (plus more) platform — doing what traditional "gold standard" tests do cost-effectively, reliably, and at scale.
Bionano is well-positioned to be the Illumina of mapping technology, which will eventually replace existing chromosome-level (cytogenetic) tests entirely and support identifying new therapeutic targets.
However, their success heavily relies on third-party labs pivoting away from legacy techniques they're already using to Bionano's system.
Understanding the human genome is like understanding a language. Before you learn how to write well, you need to know how to read. So before we get too carried away with gene editing and precision medicine, let's first ask the fundamental question — how do we read DNA?
What's confusing to most people is that there are many ways to read DNA. Each method serves different purposes, like separate tools in a toolbox.
Different technologies also determine the level of granularity you can read the genome. To use an analogy — different types of planes fly at different cruising altitudes:
If you're flying across a city, the altitude you're at determines how well you can make out what's below you. In recent years, much attention has been focused on refining the under 10,000 feet view through DNA sequencing by companies like Illumina.
But equally important is reaching a 40,000+ feet view — and that's what the field of "cytogenetics" does. What's peculiar about cytogenetics is that the tools used today are the same antiquated tools used decades ago — karyotype, microarray, and FISH.
Bionano (BNGO) is like a turbojet that can fly at a wide range of altitudes — from 15,000 to 50,000 feet. It offers a Swiss Army knife solution by combining all three traditional tests in one platform and can detect things that existing methods would otherwise miss. The technique used is called "optical genome mapping."
I was initially drawn to dig deeper because I've heard very little talk about optical genome mapping in my network thus far, being trained in clinical genetics myself. I wanted to know — what's this all about and why aren't more people talking about it yet?
To understand the company, how their technology works, and how it can be implemented in clinical practice, we'll work our way up:
What is optical genome mapping?
OGM may eventually replace gold standard cytogenetic tests
Why genome mapping and sequencing are synergistic (not competitive)
How Bionano can be used in clinical settings
Challenges to overcome
The big picture
What is optical genome mapping?
Bionano uses optical genome mapping (OGM) to detect large changes in the genome called "structural variations." Sticking with the plane analogy — imagine you're flying across the San Francisco Bay Area. Structural variations are things you can see that shouldn't be there — maybe the Golden Gate Bridge is missing, or there's a huge island that emerged out of nowhere.
There are many different types of structural variations:
Sometimes, having these structural variations can cause disease — like rare neurodevelopmental disorders or certain cancers.
OGM works by first isolating DNA from the sample cells. Then, fluorescent tags bind to sites along the genome, creating unique patterns for each section of DNA. These fluorescent tags are like bulbs on string lights, unevenly spaced apart.
The DNA is then loaded into a chip for imaging where it uncoils, like untangling a very long strand of string lights. Finally, algorithms convert these images into a "genome map."
Bionano visualizes the data using these funky-looking circular plots. Here's an example of a tumor cell with lots of structural variations (left plot) and a normal cell with not as much going on:

If cytogenetics is brand new to you and you want to learn more, I found Bionano's COO Mark Oldakowski's talk a great introduction to OGM.
OGM may eventually replace gold standard cytogenetic tests
So you might be wondering, okay cool. Fancy circle plots. But why is this such a big deal?
OGM is catching field experts' attention because it can do what all three traditional cytogenetic tests do really well— cost-effectively, reliably, and at scale.
Here's why:
Bionano's current cost per genome is ~$450, expected to be less than $100 by 2023. That's comparable, if not cheaper, than existing tests.
Resolution gets down to 500 base pairs (and maybe even better in some areas), with false positives below 2%. In comparison, microarrays only get down to around 50,000 base pairs, karyotypes in the 5-10 million range.
Variants detected even in small samples — Single-molecule imaging means that Bionano can detect variants present in as little as 1% allele fraction (percentage of the sample with this variant). This is useful in cancer testing because cancer cells are often heterogeneous, with some cells having certain mutations, others having another type of mutation(s).
Shorter turnaround time for results and less labor-intensive — 3 samples require less than 3 minutes of hands-on lab technician time. You don't need to be a biostatistician to analyze the data because the software does all the heavy lifting. One machine can run 96 samples per week at 100x coverage (number of times the genome is read).
So far, clinical studies have shown Bionano has 100% concordance with traditional cytogenetic methods. What's more, it appears that OGM's higher resolution technology is also picking up structural variations that would have been missed by karyotype or microarrays. For example, this study found new clinically relevant information in 11% of leukemia cases that routine methods had missed.
As you fly at a lower altitude, you get to see landmarks below you more clearly, picking up things you wouldn't see at a higher altitude.
Alex Hoischen focuses on immuno-genetics research and put it this way:
"Optical genome mapping could potentially replace all of those tests [karyotype, microarray, FISH] in one go. If the average leukemia cell gets 5 or so tests, we could just replace it with a single test. You get the same results, or potentially even more complete, faster."
[Mendelspod: Alex Hoischen, timestamp 11:10]
Dr. Brian Levy, a cytogenetic expert from Columbia University, said he "recommends that OGM be considered as a first-line test for detection and identification of clinically relevant structural variations."
The bottom line: Bionano is still for research use only, but OGM shows promise in eventually replacing all gold standard cytogenetic tests in the clinic entirely — similar to how next-generation sequencing replaced Sanger sequencing in recent years.
Why genome mapping and sequencing are synergistic (not competitive)
I keep seeing investors trying to compare Bionano with Illumina (short-read sequencing) or Pacific Biosciences (long-read sequencing).
This is an unfair comparison. Bionano doesn't compete or substitute any sequencing technology. In reality, genome mapping complements sequencing by putting the pieces together more accurately. Here's what I mean:
Sequencing works by chopping up DNA into smaller pieces and then sticking them back together again. It's like having many redundant puzzle pieces that you need to sort through and put back together. Long-read sequencing creates bigger, fewer puzzle pieces compared to short-read sequencing — which increases costs but decreases the chance of missing stuff:
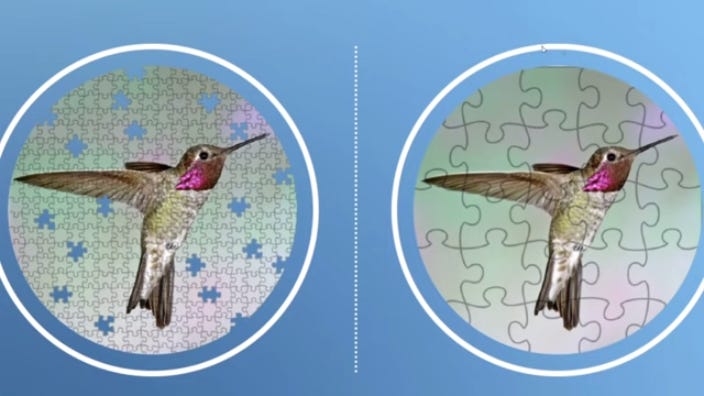
Genome mapping is like having the reference picture on the puzzle box in front of you. It makes it easier to know where to put each of the puzzle pieces correctly.
If you're really into analogies, here's another one describing the difference between OGM and sequencing using physical location descriptors. Go knock yourself out:

Combining genome mapping and sequencing is known as "hybrid scaffolding," and it's becoming increasingly more common. Some examples:
OGM + long-read sequencing: The Vertebrate Genome Project aims to sequence the genomes of 70,000 species — they use genome mapping to aid with the accuracy of their sequence calls.
OGM + short-read sequencing: Structural variations are commonly found in cancer cells — but can't be detected using short-read sequencing alone. This study showed that you could combine short-read sequencing with OGM for lung cancer patients. Two new oncogenes were discovered by using hybrid scaffolding.
Okay, but can structural variations be picked up without OGM?
Possibly, if computational methods improve and the cost of long-read sequencing decline substantially. But thus far it's proven to be challenging and expensive. PacBio can only detect 72% of structural variations Bionano picks up. What's more, PacBio's method costs $10-20k per genome, whereas OGM is less than $500. So it feels like OGM is here to stay for some time.
George Church, professor of genetics at Harvard and co-founder of 20+ biotech companies, commented on Bionano:
"The Bionano optical genome mapping system provides direct and unambiguous identification and characterization of large-scale DNA structural variants without complex bioinformatics. [Sequencing] helps zoom in on low-frequency variants with single-base resolution. This seems a great match to provide genomics and clinical researchers with the gamut of DNA variant information from large to small."
How Bionano can be used in the clinic
The clinical utility of OGM is still TBD — identifying use cases for this new tool is an active but nascent area of research. Bionano already has several clinical research partners who seem to love what the new technology can enable. I could write an entirely separate post on this section, but here are some high-level thoughts:
Cancer is and will likely continue to be a prominent use case for OGM.
This is a bit unintuitive to grasp, but 100% of cancer cases are genetic — in the sense that there are always genetic aberrations in cancer cells. Some are structural changes like rearrangements or inversions, which can't easily be picked up by gene sequencing. OGM can be used to diagnose more patients faster and identify potential new gene targets for new cancer therapies.
Diagnosis of repeat expansions disorders like Fragile X via OGM could be big, if possible.
Without getting too in the weeds here — having the capability to diagnose repeat expansion disorders means Bionano goes beyond replacing cytogenetic testing. Bionano has a patent that allows them to get better than 500 base pairs resolution for some genome regions. Like having a special magnifying glass for hotspot areas.
This is valuable because repeat expansion disorders cannot be detected by these methods: karyotype, microarray, FISH, sequencing. You need a separate test.
Bionano's technology has already shown success in diagnosing facioscapulohumeral muscular dystrophy (FSHD) to replace the gold standard method, Southern blot analysis.
If extended to other repeat expansions disorders like Fragile X, I can see this being useful in the pediatric (maybe prenatal) setting — where microarray and Fragile X tests are already commonly ordered (separately) for children with neurodevelopmental delays or autism.
Embryo screening for chromosomal abnormalities is an interesting application of OGM.
I haven't yet seen any studies or groups creating preimplantation genetic testing for aneuploidy (PGT-A) and structural rearrangements (PGT-SR) using OGM. I'm not sure if there are any technical limitations, but given over 40% of all IVF cycles include PGT, it's a big market.
Existing methods for embryo testing use either microarray (Natera) or sequencing (Invitae, Igenomix, etc.) Structural variations can be easily missed or incorrectly reported. I wonder if OGM can help solve these issues.
The caveat with ALL of this is that: more information is not within itself better. So as labs are developing tests using OGM, they need to be mindful of improving the patient experience, not merely because it's technically feasible. That means having a high threshold in reporting structural variants that can impact human health, not just variations that many of us likely walk around having — but we don't know about because we haven't gotten tested.
Challenges to overcome
The biggest challenge I see for Bionano is achieving acceptance of OGM utility within the scientific and clinical community. Justifying switching costs from legacy techniques to OGM is a massive undertaking that can take labs a long time.
You may have noticed this piece is light on analyzing the company's business. That's because it's been difficult for me to properly grasp the scope and scale of the addressable market. I stared at their TAM slide for a long time and struggled to see how they could meet this in the next few years based on sales performance to date.
As of June 2021, Bionano has only shipped 121 systems after 4 years. This is a far cry from the projected 10,000 systems market size — though it's possible for them to scale fast given the right conditions. It looks like they view hybrid scaffolding (enhancing sequencing data in the R&D setting) as a slightly bigger market than replacing existing cytogenetic tests.
Bionano's long-term strategy for driving widespread clinical adoption is by proving the value of optical genome mapping through clinical studies. This will support insurance reimbursements (which they have recently gained headway on) and professional guidelines recommending the use of OGM in clinical practice.
All makes sense — except adoption, and hence, Bionano's success heavily relies on the speed in which labs choose to pivot to Bionano compared to legacy techniques they're already using. As Bionano's CEO Erik Holmlin put it:
"It's really up to the labs to have their plan to develop an assay, which involves picking an indication... They set the cutoffs for what determines a positive or negative result in the assay, and they determine how they're going to report that to their ordering physicians. And then they validate that assay by running known samples against a gold standard, which is typically the traditional methods, karyotyping, fish and microarrays. And once they determine that they've met the validation criteria, then they can make that assay available on their test menu... and ordering begins at that point." [Bionano Q2 2021 Earnings Call: Erik Holmlin]
That's a lot of upfront time and effort that third-party labs need to decide whether it's worthwhile for them to put in. Even if data on Bionano's value add is super compelling, it still may not convince everyone.
The big picture
Overall, I believe there will be a massive adoption of genome mapping tools in clinics within the next 5 to 10 years. OGM will likely replace all existing cytogenetic tests entirely — similar to how next-generation sequencing replaced Sanger sequencing in recent years.
Concurrently, genome mapping also complements sequencing efforts. Thus, as the sequencing market grows, OGM will likely also grow. If Bionano continues to be the only one offering OGM reliably and at scale, it can become the Illumina of optical imaging.
At the same time, sales and revenue trajectory is somewhat unknown — it takes time to convince labs to switch technologies, even if the benefits are apparent. Bionano has historically struggled to find buyers for its product, but hopefully, the expansion of its commercial leadership team will help accelerate growth.
I didn't feel the need to put disclaimers in my previous posts, but an unsavory interaction with someone I won't name obligates me to remind you all that it is never my intention to "pump and dump" stocks I write about. (I don't have sufficient influence to do so anyhow 😂 )
Bionano is on my watchlist, but I don't currently hold a position. I have no financial incentives or professional relationships with Bionano or other companies that I have researched thus far.
I genuinely am just sharing these posts because I write to learn and thought others might find it interesting or helpful, too. So for those getting the wrong idea — take a chill pill. Please.
On that tangent — what am I wrong about or what might have I missed? If you’re finding this newsletter interesting, share with me your feedback; you can respond to this email or tweet at me.
I would also love it if you share this piece with your network and subscribe if you’re new here.
Thanks for reading!
Christina









Very cogently explained for people without background in genomics. Thanks !
https://www.yahoo.com/now/bionano-biodiscovery-acquisition-further-bolsters-160041653.html