Curing Type 1 Diabetes — Making stem cells produce insulin
Highlighting key players on a quest to reverse type 1 diabetes with stem cell-derived islets
Welcome to Health & Wealth — your weekly source of the latest health research and biotech trends. I tabled my research on this topic a few weeks ago but thought it was worthwhile to revisit and synthesize through writing. If you are new, you can join here and share within your network — word-of-mouth is how I grow. Feedback is encouraged and welcome. Happy reading!
Article Highlights
One hundred years ago, Type 1 Diabetes — an autoimmune disease that destroys the body’s insulin-producing cells — became treatable. But the economic and psychosocial burden of disease remains high. Costs for an adult with T1D are ~$20,000 annually.
Now researchers are working to reverse Type 1 by replacing the missing cells. Last fall, a NY Times article about a man cured of Type 1 took center stage, sparking promise of an imminent stem cell therapy.
Two main challenges to solve for include 1) creating a renewable source of beta cells and 2) protecting them from immune attack to prevent the need for immunosuppressants.
Notable public companies working on T1D cell therapies include Vertex Pharmaceuticals, CRISPR Therapeutics in collaboration with ViaCyte, and Sana Biotechnologies.
For many with diabetes, insulin is a lifeline — it made Type 1 Diabetes (T1D) a treatable, rather than terminal, disease. But insulin is by no means a cure. Managing T1D involves constant vigilance with dependence on injecting or pumping insulin for life. A mother I spoke with describes life with a husband and 4-year-old son with T1D:
“As a parent, you never stop thinking about it and you don’t just get to check out… Henry does have extra needs because he has diabetes. We’re up with him in the night still sometimes. It’s like having a baby, forever, and I don’t know if I’ll ever get consistent and regular full nights of sleep.”
Today we’ll explore current progress on an alternative approach: what if living cells, instead of machines or injections, were used to control blood glucose?
Instead of supplying the body with insulin, is it possible to replace the beta cells that make insulin — effectively curing the disease?
The economic and societal benefit of a cure is clear — the annual cost of T1D is more than $90 billion globally, with ~18 million people in the world affected. I’ve long been interested in regenerative medicine but assumed progress towards a T1D cure was still in the intangible, distant future. It wasn’t until last fall, when I came across a NY Times article about the first person presumably cured of Type 1 diabetes did I begin to seriously investigate this space.
Turns out there’s an entire underworld racing to cure diabetes within the next decade. Notable public companies working on T1D cell therapies include Vertex Pharmaceuticals (VRTX), CRISPR Therapeutics (CRSP) in collaboration with ViaCyte, and Sana Biotechnologies (SANA), along with several others in the running.
Yet some in the T1D community are understandably skeptical. They’ve been promised a cure is on the horizon for ages. The joke has been that it’s always “just five years away” — like a boy who cried wolf too many times. What makes now any different?
To understand why we’re at an inflection point and how a functional cure will come to fruition, I invite you to come down the rabbit hole with me:
Two main challenges to overcome: supply and protection
Key players’ approaches and progress
Vertex Pharmaceuticals
ViaCyte & CRISPR Therapeutics
Sana Biotechnologies
The big picture
Two main challenges to overcome: supply and protection
In people with T1D, the body attacks its pancreatic cells that produce insulin by mistake. You might not know this, but there’s already a viable cure to Type 1 Diabetes. It involves patients receiving islet transplantations (or whole pancreas transplants) from cadavers.
One glaring problem: there will never be enough pancreas organ donations to cure everyone with T1D. So how can we develop a renewable source of beta cells?
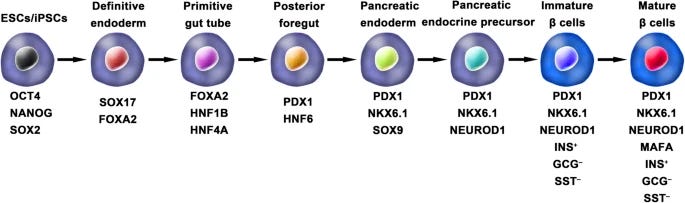
The answer lies in creating stem cell-derived cells, which can theoretically generate endless quantities of beta cells and solve the supply issue. Stem cells are coaxed through a series of differentiation steps to become mature beta cells:
Patients would then receive an infusion of these cells to replace the beta cells they lacked.
But that only solves half of the problem. Because you see, a universal problem with all “off-the-shelf” cell therapies is that they can be rejected as foreign when introduced to the body, resulting in the elimination of the transplanted cells.
One workaround is to take drugs to suppress the immune system. But lifetime immunosuppression can pose more risk than reward. Plus, only those with a severe risk of hypoglycemia would be eligible for transplant.
That leads us to the second challenge: how can beta cells be protected from immune destruction without the need for immunosuppressants?
This is where companies diverge the most in their approaches. I’ll describe more in detail the mechanisms as we review each company’s progress, but in broad strokes, you can:
Package the cells in a device to physically protect them
Modify the cells so they become “invisible” to the immune system
Teach the immune system not to attack introduced cells
Of course, the beta cells also need to stay functional in the body for years or even decades.
Now that we’ve mapped out the problems to be solved let’s turn our attention to the progress being made by several companies.
Vertex Pharmaceuticals
Vertex currently receives the most limelight because they released early positive Phase 1/2 data for the first patient dosed with VX-880, a stem cell-derived therapy for T1D. The patient was Brian Shelton, whose story was profiled in the NY Times. The amount of daily insulin he was taking 90 days after the infusion has dropped by 91%, and measures indicate restored glucose-responsive insulin production:
Vertex uses a differentiation protocol established by Doug Melton and colleagues at Harvard, who had been working on steering embryonic stems to becoming islets for over 20 years. Melton started a company Semma in 2014 to scale the growth of islet cells, which was later acquired by Vertex for $950 million in 2019.
But data from one patient is premature, and the trial is still ongoing. What’s more, VX-880 requires chronic immunosuppressive therapy to protect the islet cells from immune rejection.
They’re working on another program that would encapsulate the islet cells, creating a physical barrier that would block immune attack. This could remove the requirement for immunosuppression, though few details of this preclinical effort are disclosed.
ViaCyte & CRISPR Therapeutics
ViaCyte provides some more insight into what an encapsulation device might look like. Their first-generation product is a mechanical “pouch” that allows blood vessels to enter and interact with implanted cells. This is implanted under the patient’s skin to act as a surrogate pancreas that releases insulin.
It’s like a teabag — allowing the contents inside to “steep” by receiving oxygen, nutrients, and glucose while releasing insulin:
Unlike Vertex, ViaCyte uses pancreatic progenitor cells — a more immature cell type that is hoped to later mature into functional beta cells after implanting into the body. This doesn’t seem ideal. Compared to fully differentiated cells, progenitor cells add a layer of variability and uncertainty, plus months lag for functional cells to emerge after implantation.
In 2018, ViaCyte penned a deal with CRISPR Therapeutics to take their science a step further. Their hope is to gene edit the cells to evade immune system detection while still using a version of their original pouch device. In February 2022, they announced they dosed their first patient with VCTX210 for their Phase I trial.
My understanding of how they plan to protect cells from immune rejection is to express PD-L1, which helps protect the cell from T-cell attack while removing MHC-I. This critical protein serves as an “identification tag” the immune system uses to recognize foreign versus self.
I’m having trouble understanding why a capsule is still needed if cell modification works to evade immune detection. This, combined with using immature progenitor cells, gives me pause about Viacyte/CRISPR Therapeutics’ strategy.
Sana Biotechnologies
Sana Biotechnologies is perhaps the dark horse working on truly cloaking stem cell-derived islet cells from the immune system. It’s called “hypoimmune therapy” — introduced cells don’t get recognized and degraded by the body, effectively like Harry Potter donning his invisibility cloak, flying under the radar.
Sana is a platform technology working to disrupt MHC protein expression and overexpress CD47, a protein that hides cells from the innate immune system, including macrophages and natural killer cells. This method can be used to create a variety of off-the-shelf cell therapies, including modified beta islet cells. The goal would then be to implant these modified cells directly into the pancreas to work like normal healthy islet cells.
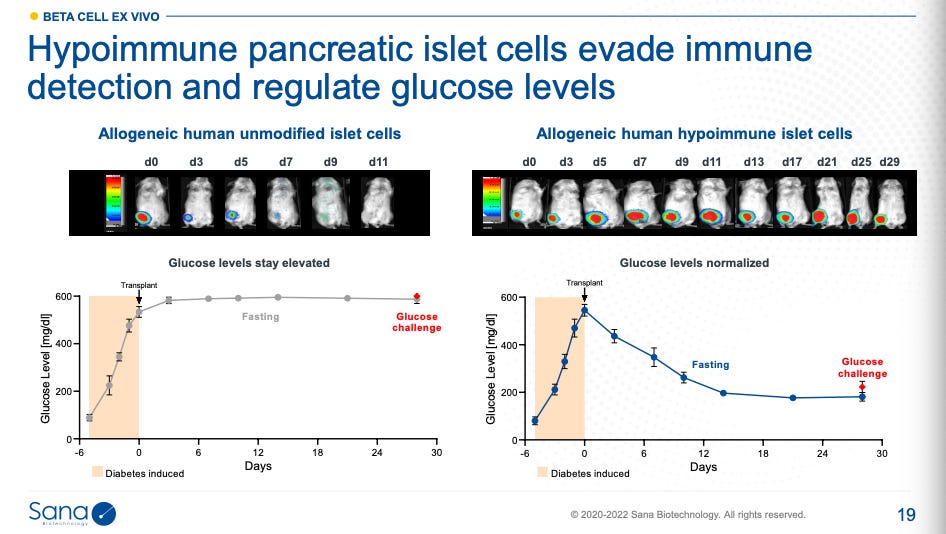
In mice, hypoimmune therapy modifications have shown functional restoration of glucose regulation. Without these edits and immunosuppression, the introduced islet cells don’t survive (data on left), and thus insulin production isn’t sustained:
Their diabetes program, SC451, is still in preclinical development, and IND submissions for human clinical trials are not expected until later this year or next. Nevertheless, I believe this approach is promising.
The big picture
In many ways, Type 1 Diabetes is one of the lowest hanging fruits in regenerative medicine. The condition selectively attacks a single cell type, so it’s clear what needs replacing. You also don’t need much (beta cells only account for 1-2% of the mass of the pancreas) and it can be placed elsewhere, like under the skin, as long as it has access to the bloodstream.
Early evidence is emerging as therapies progress in clinical trials. There’s still work to be done, but it’s become clear we’ve reached a tipping point. It’s no longer a question if stem-cell transplant for T1D is possible, but when.
My sense is that first-generation therapies that require chronic immunosuppression won’t gain significant traction even if approved. An effective solution to protect these engineered cells from immune attack will ultimately win out.
Further reading
A longstanding partner in accelerating T1D cell therapy research is JDRF. This JDRF talk highlights a few other methods I didn’t mention, including microencapsulation and scaffolds.
A review article about the space written by Doug Melton, who led efforts on the islet cell differentiation protocol used by Vertex Pharmaceuticals.
If you enjoyed this post, subscribe and tell your friends!
You can also follow me on Twitter @healthwealthgen for the latest updates.
My publishing cadence has slowed as I’ve been poking around areas I’m less familiar with, including DNA synthesis and AI drug discovery. If you have insights or expertise in this area, please reach out.
Thanks for reading!
Christina