Emerging trends in genetic counseling and personalized medicine
Why genomics is a moving target with an evolving intersection between what's scientifically possible and ethically feasible
Hi friends 👋
Welcome to Health & Wealth — your weekly source of the latest health research and biotech trends. For those in the states, I hope your hearts and bellies are full from Thanksgiving festivities last week 🍁 💛
I recently gave a talk for a healthcare conference in Taiwan on key trends in genetic counseling and personalized medicine and where the future is headed. Thought it was relevant enough to share here on Health & Wealth.
No specific company analysis today, but here is a lay of the Genomics Land from the eyes of a genetic counselor!
Article Highlights
Genomics is at an inflection point in which the pace will only accelerate — we're in the first inning with a long run ahead of us. Here are some of my predictions:
Liquid biopsies will be used as first-line screening and diagnostic tests. The recent non-invasive prenatal test revolution has shown us that solid science applied in the right market leads to rapid commercialization and growth.
Reproductive technologies will increasingly play a role in how people build their families. Preimplantation genetic testing will continue to grow as awareness and access to IVF grow.
Genetic risk predictions for common diseases will improve. Clinical genomics will soon integrate monogenic risk variants with an individual's polygenic background.
But genetic tests are just tools to get more information. What matters for people is to make meaning of this new information. That's where genetic counselors come in.
Having some trouble getting a hold of my recorded talk, but here are some written highlights. Screenshots here are pulled from my slides. We’ll touch on:
How genomics is still in its nascent stage
What’s next: 3 emerging trends in clinical genomics
The importance of genetic counseling
Genomics is still in its nascent stage
Even insiders in the genomics field can appreciate that we're still in the early innings. We're at an inflection point in which the pace will only accelerate.
Numerous factors culminate to put us at the cusp of pulling everything together. These include the drop in genomic sequencing cost, increasing access and breadth of genetic testing, and improvements in tools to help us better understand what genetic variation means for human health.
In short, it's an exciting time to be part of the field — both as a professional and investor.
What’s Next: 3 Emerging Trends in Clinical Genomics
The field of genomics is large, and there will be many players and innovations. By no means a comprehensive list, but here are 3 trends I have some insight on and believe will shape clinical genomics as we know it.
1. Liquid biopsies will be used as first-line screening and diagnostic tests.
Liquid biopsies are molecular-level analyses of bodily fluids such as blood, saliva, and urine. But why should we care — what problems do they solve?
Many current diagnostic tools remain expensive and hard to access. If you think of imaging technology like MRI or CT scans or diagnostic procedures like amniocentesis — these require specialized equipment and highly-trained specialists. Moreover, they're often exclusively found in large hospital institutions, not local family clinics.
Liquid biopsies can remove some of these barriers by lowering costs and increasing access. Blood draws are much easier.
Liquid biopsies can be used for a variety of applications, including in prenatal and cancer care.
Non-invasive prenatal testing (NIPT)
NIPT (or cell-free fetal DNA) is a blood test that screens for chromosomal abnormalities in the baby. Specifically, it works by detecting pieces of the baby’s DNA circulating in the mother’s bloodstream during pregnancy.
NIPT is a successful example of how liquid biopsies fundamentally changed clinical practice in a very short period. It became commercially available in 2011. Today, it is recognized by the American College of Obstetricians and Gynecologists as a universal screening option for all pregnant women and is routinely ordered by doctors.
As a result, the volume of prenatal diagnostic procedures (amniocentesis, CVS) has declined. Clinics often avoid using the words "invasive" procedure to not actively discourage patients from electing to do amniocentesis or CVS.
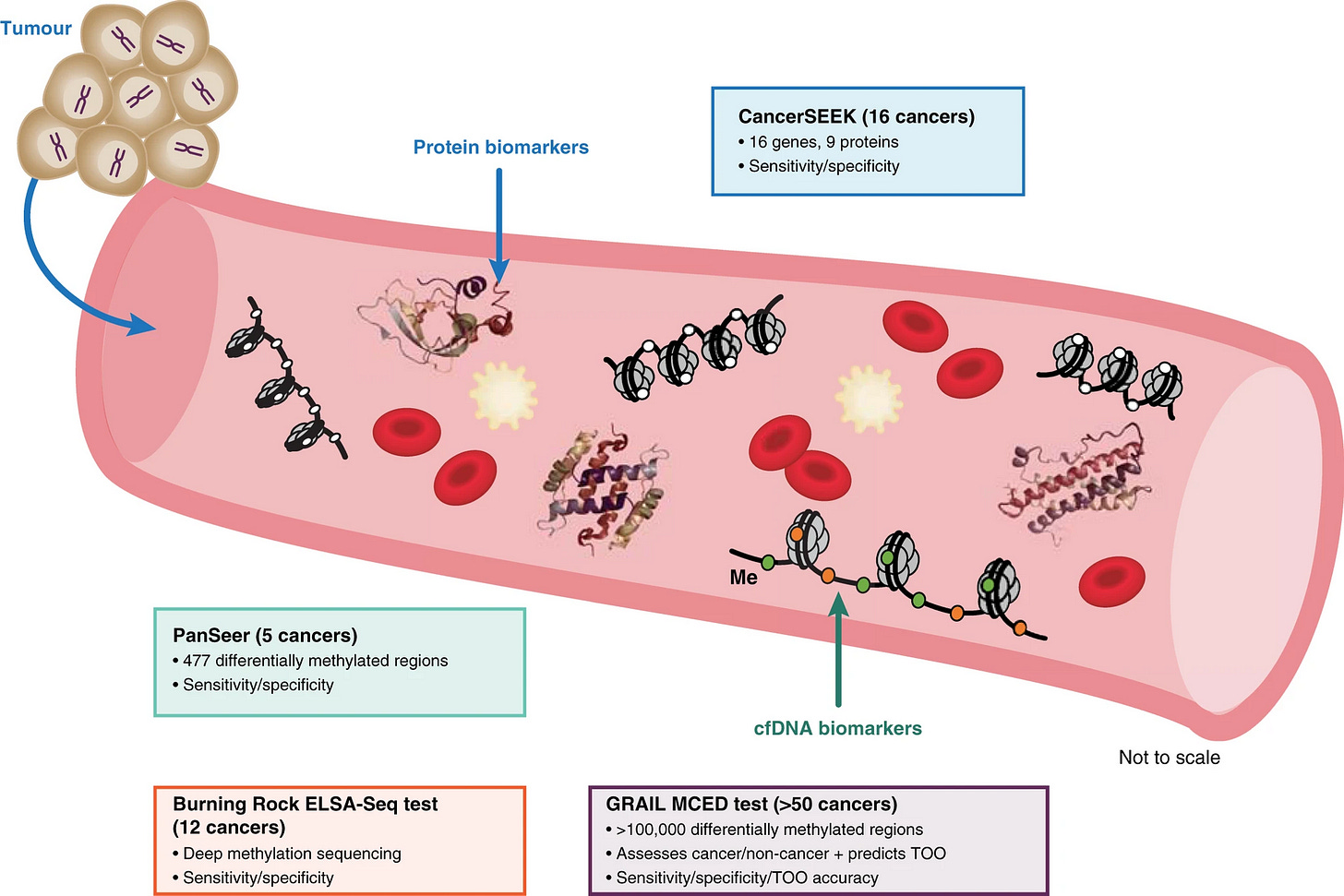
Multi-cancer early detection blood testing
A similar transition will happen for cancer screening blood tests. Using technology similar to NIPT, cancer blood tests like GRAIL can now screen for up to 50 different types of cancer. The aim is to detect cancer earlier when it can be cured to improve patient outcomes.
We're still in the early days of clinical adoption, but I believe the transition to using cancer liquid biopsies will happen faster than we might think.
The recent NIPT revolution has shown us that solid science applied in the right market leads to rapid commercialization and growth.
2. Preimplantation genetic testing will continue to grow as awareness and access to IVF grow.
Patients going through in vitro fertilization (IVF) have the option to genetically test their embryos created in the lab before transferring into the woman's uterus. This type of genetic test is known as "preimplantation genetic testing" (PGT).
Rise and adoption of preimplantation genetic testing (PGT)
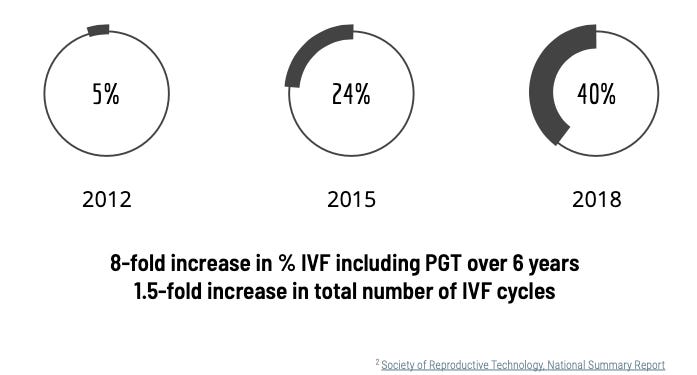
In the United States, the overall growth of IVF has slowly increased, but what's much more striking is the growth in PGT.
The latest final data tell us that in 2018, about 40% of all IVF cycles included some form of PGT in the US. That's an 8-fold increase in just 6 years.
Many factors contribute to this trend, including the desire from patients and doctors to implant chromosomally normal embryos to decrease the chance of miscarriage and time to pregnancy. The benefit of PGT for all IVF patients is still hotly debated amongst experts, but it is clear this trend is here to stay.
There is still a long way to go to truly make IVF accessible and cost-effective. Innovation is happening on multiple fronts in this space. I will likely write future pieces on this because reproductive medicine is my home turf. In the meantime, David Sable is a level-headed, sage voice on charting the path forward in IVF. Highly recommend checking out his writing.
3. Genetic risk evaluation will soon integrate monogenic risk variants with an individual's polygenic background.
Okay, before I get any further on this point, some definitions.
A monogenic condition is caused by variation in a single gene. This faulty gene works in a way that is not typically intended. A polygenic condition is due to a collection of many genetic variations in many genes. The majority of diseases we're familiar with are polygenic. I see "polygenic" thrown around in the wrong context a lot. It happens to the best of us — I recently pointed this out to an ARKG analyst.
A way to capture polygenic background is through polygenic risk scores (PRS). PRS aims to estimate an individual’s genetic risk of developing a polygenic disease based on their unique genetic variations. This is done by assessing the cumulative effect of many common variants into a score.
Utility of polygenic risk scores (PRS)
Once again, we are in the early days of PRS. But the idea is to identify individuals with a higher risk of developing certain diseases like heart disease. Knowing ahead of time can influence clinical management and personal health behaviors.
Monogenic and polygenic risk aren't mutually exclusive; they're likely synergetic.
There seem to be two camps emerging — those who believe clinical genetics should remain focused on what we know well (monogenic and rare genetic disorders) and others who want to build towards a future using polygenic scoring. What if this is a false dichotomy?
Those in clinical genetics know the concept of "variable expressivity" and "reduced penetrance" — when rare genetic mutations don't impact everyone the same way. Why is that?
Part of the explanation can be that monogenic variants don't show the full picture. There may be other genetic factors at play that we've historically missed. And a missing piece of the puzzle is polygenic risk.
I believe in a future in which the two worlds — monogenic and polygenic — will merge. They are not mutually exclusive. Polygenic risk can likely further modify risk and explain the variation in patient outcomes.
Let me give you an example of this and what we're already beginning to understand.
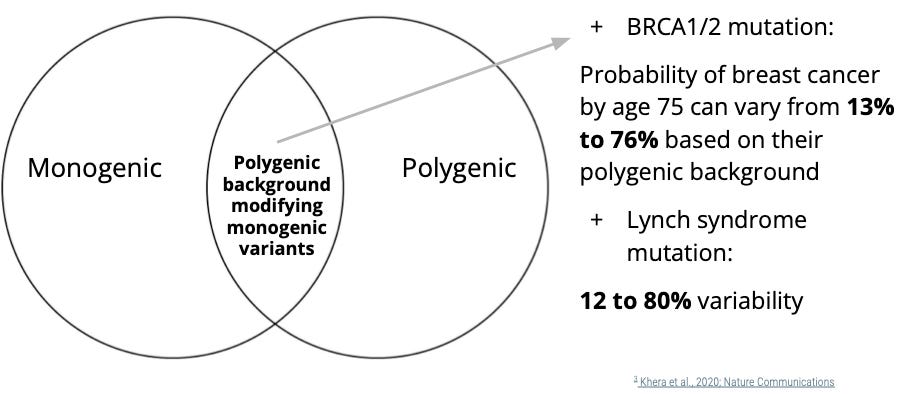
Having a mutation in a cancer susceptibility gene increases your risk of developing certain types of cancer. But by how much? Today, we usually give patients a broad range, factoring in their personal and family history.
But a recent study found individuals with a BRCA1 or BRCA2 mutation have vastly different probabilities of developing breast cancer based on their polygenic background.
If they have a BRCA1/2 mutation but have a low PRS for breast cancer, their risk can be as low as 13%. But if they have a "double whammy" — BRCA1/2 mutation plus a high PRS for breast cancer, their risk can be as high as 76%.
The same is true for Lynch syndrome mutations — a type of hereditary colon cancer.
What does this mean? It means that it's not inconceivable for future cancer clinics to also measure polygenic risk in addition to hereditary gene panel testing. Because even if someone has a cancer gene mutation, it behooves the doctors and patients to know whether their cancer risk is much higher or lower than expected.
How will individuals receive PRS?
Much of the debate about PRS is on the technical feasibility of PRS — how its generated, its predictive ability, its applications.
But equally important is discussing how this new type of genetic information is communicated to people and how individuals respond to knowing their probabilistic susceptibility to disease.
Genetics is rarely a guarantee in binary outcomes.
Some are concerned that people will misinterpret the information presented to them:
But is this actually true about how people will respond? I happen to think that any information can be misinterpreted out of context. I’m also optimistic that with the right resources and guidance, these concerns can be minimized.
Importance of genetic counseling
With all these rapid changes in technology, what doesn’t change is human psychology and human nature. There will always be uncertain and difficult situations to face.
Clear communication and genuine empathy are vastly underrated.
At the end of the day, genetic tests are just tools to get more information. What matters for people is to make meaning of this new information.
And that’s what makes genetic counselors critically and irreplaceably important. They are master communicators of complex information and facilitators of complex human emotions. Hope I’m doing my profession justice here on Health & Wealth 😉
What am I wrong about or what might have I missed? Your feedback helps me make this great. Reply to this email or tweet me @healthwealthgen
Getting my booster shot tomorrow. Wish me luck🤞
Christina