MaxCyte's snowball effect: Riding on the shoulders of top CRISPR companies
How MaxCyte makes revenue from cell-therapy companies like CRISPR Therapeutics, Editas, and Caribou's R&D expenses
Hi friends 👋
Welcome to Health & Wealth — your weekly source of the latest health research and biotech trends. For those who have joined, thanks for being early adopters, you guys are awesome! 😎 If you are new, you can join here and tell your friends. Feedback is encouraged and welcome. Happy reading!
Article Highlights
MaxCyte is a company that you probably haven't heard of, but you should. They make machines to deliver gene editors (and other things) into cells through a process called electroporation.
This is a big deal — MaxCyte's machines allow cell therapy companies to mass-produce cells that have been gene-edited outside of the body (ex vivo) for therapeutic use.
At present, this is a de-risked way to capture the over $250 billion cell therapy revolution because you get the upside of cell therapy market growth while diversifying risk across a dozen, potentially hundreds, of therapies.
However, ARK Invest isn't bitting likely because scientists are developing new approaches that could bypass the need for ex vivo treatments entirely.
Unless you've been hiding under a rock, you've probably heard of CRISPR. It's the molecular scissors that can be used to edit DNA. But what you might not appreciate is that the key hurdle of unlocking CRISPR's potential is actually getting the editors to go where they need to go — inside of target cells.
So a critical question for the success of CRISPR therapies today is: how can you cheaply, quickly, and reliably deliver CRISPR components into a lot of cells to do its gene-editing magic?
Today we're going to be talking about a company that does just that. Simply put, MaxCyte (MXCT) makes machines that let you put things inside of cells. The delivery strategy used is called "electroporation."
In the context of CRISPR therapies, MaxCyte allows companies to mass-produce cells that have been gene-edited outside of the body. The cells then get introduced into patients through their bloodstream for treatment. Here are some compelling numbers from their deck in January 2021:
MaxCyte supports all stages of clinical development, from research to commercialization. On the cell therapy side, they have 140+ partner programs, with 100+ being licensed for clinical use. On the drug discovery side, all of the top 10 global pharma companies use MaxCyte.
Many of the top gene-editing companies use MaxCyte. These include pioneers like CRISPR Therapeutics, Editas, and Caribou Bioscience, to name drop a few. MaxCyte is the gold standard in delivery systems for ex vivo treatments.
As gene editing (and other cell therapy) companies grow, MaxCyte also grows due to their layered revenue model:
License fees of $150K/year a machine during clinical trials and $250K/year once the company enters the clinic. Companies also need to buy $200-1,500 single-use disposables to keep using these machines.
Milestone payments as companies progress through clinical trial phases over time (all pre-commercial) are worth over $950 million.
Royalty deals for therapies that have successfully commercialized.
On top of that, they have high recurring revenues (72%) and high gross margins (89%).
To understand the company, how their technology works, and how it can be implemented in clinical practice, we'll work our way up:
What is electroporation?
Which is the better delivery strategy: electroporation vs. viral vector
MaxCyte’s snowball effect
Potential risks and unknowns
The big picture
What is electroporation?
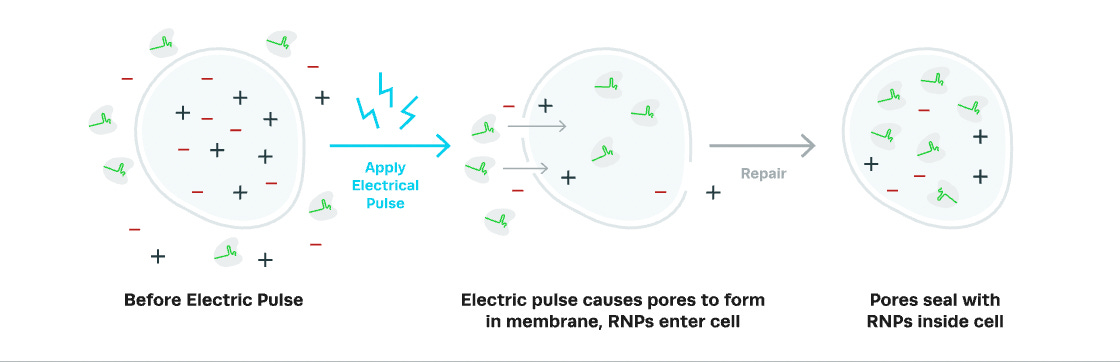
The process of putting things inside of cells is called "transfection." There are several different ways to do this, but MaxCyte uses electroporation to create temporary holes in the cell membrane to allow stuff to pass through.
The technique applies an electrical field to cells which opens up pores in the cell membrane. The openings allow the entry of macromolecules like DNA, RNA, and protein into the cytoplasm. This allows cells to uptake Cas9 mRNA (instructions to create the molecular scissors) and sgRNA (guides to tell the scissors where to go). Once the electrical field is removed, the membranes can reseal, trapping the editors inside.
Electroporation is like steaming your face before a facial — it opens up your pores and allows skincare products to better absorb into your skin. Or so I'm told.
Which is the better delivery strategy: electroporation vs. viral vector
For commercial use, the most common delivery strategies for CRISPR used today are electroporation and viral vectors.
Most strategies for non-viral delivery of genome editing tools rely on electroporation (at least for now). So really, for all intents and purposes, "non-viral" means electroporation. And MaxCyte does electroporation really well — reliably and at scale.
On the other hand, viral vectors involve packaging the sgRNA/Cas9 sequences into non-replicating viral particles. And we know viruses are very good at getting themselves into cells. So viral vectors are like cargo trucks delivering the goods.
So you might be wondering: okay, so which is the better gene editing delivery method? This can help us determine the current and future demand for MaxCyte's electroporation machines from gene editing companies. A battle between electroporation and viral vectors — let's go!
However, the unsatisfying answer to this question is that it depends on the use case. It's like asking, "is a screwdriver better than a wrench?"
Here are some more nuanced questions to get us closer to understanding MaxCyte's potential demand from gene editing companies:
Do you need to edit cells inside or outside of the body?
Ex vivo: Electroporation is only for editing outside of the body (ex vivo editing). It's hard to apply a strong electrical field safely to the body. But it can be used to treat genetic blood diseases (like sickle cell disease) and for cancer immunotherapies (like CAR-T or CAR-NK).
In vivo: For tissues that can't be easily replaced, like the heart, brain, or muscle, in vivo editing is needed. Viral vectors have limited capabilities to do this if the tissue is accessible by injection (like the eye) or by blood infusion (like the liver, where things end up when you infuse something into the blood). Delivery to other places of the body is still a challenge that scientists are hard at work trying to solve.
Do you need to edit a lot of cells or very few?
Electroporation is very versatile. It can be used for a single cell (like an embryo) or — with MaxCyte's machines — to mass-manufacture up to 7 million cells in seconds (200 billion cells in <30 minutes). Mass production is instrumental for faster and cheaper allogeneic "off-the-shelf" treatment.
Viral vectors are harder to scale, time-consuming, and costly. Analyst Emma Ulker describes the downsides of using viral vectors for immunotherapy:
"A single batch of lentivirus costs roughly US$500,000 to manufacture but is sufficient to treat only between 2-10 patients, and this contributes around 90% of the cost of goods of the treatment. Supply chain and manufacturing bottlenecks are another factor, and wait time to enter supply chain is currently estimated at up to 2.5 years contributing to delays in launching clinical trials, adding to cash burn and delaying treatments."
So, electroporation is the clear winner when comparing the two methods for creating treatments like edited natural killer cells for cancer treatment. It's much faster and more effective than viral vectors:

Do you need to use the patient's own cells (autologous treatment), or can you use cells from a healthy donor (allogeneic "off-the-shelf" treatment)?
Both electroporation and viral vectors can do both autologous and allogeneic therapy. Delivery methods are agnostic to where the cells came from. It is worth noting that allogeneic "off-the-shelf" therapy is considered better than autologous therapy for cancer immunotherapy because it is cheaper and more accessible. For that, electroporation is better suited for creating these off-the-shelf treatments because it's highly scalable.
Will CRISPR components work temporarily or be permanently expressed in the cells you edit (and their successive progeny cells)?
The somewhat counterintuitive thing about gene editing therapy is that you really only want CRISPR proteins to work temporarily. Otherwise, permanent expression can lead to potential safety risks — off-target effects, malignancy, and unwanted immune responses.
Electroporation is great for temporary transfection, but viral vectors can sometimes overstay their welcome, increasing the risk of off-target effects.
The bottom line: Virus vectors are good for some in vivo treatments but are expensive, time/labor-intensive, and have some potential safety risks. Electroporation is good for efficient and easy delivery for ex vivo treatments but can't be used in vivo, limiting its capabilities.
MaxCyte’s snowball effect
ARK estimates that allogeneic cells and immunotherapies could create $250 billion in incremental revenues. MaxCyte is a de-risked way to capture this market. Why?
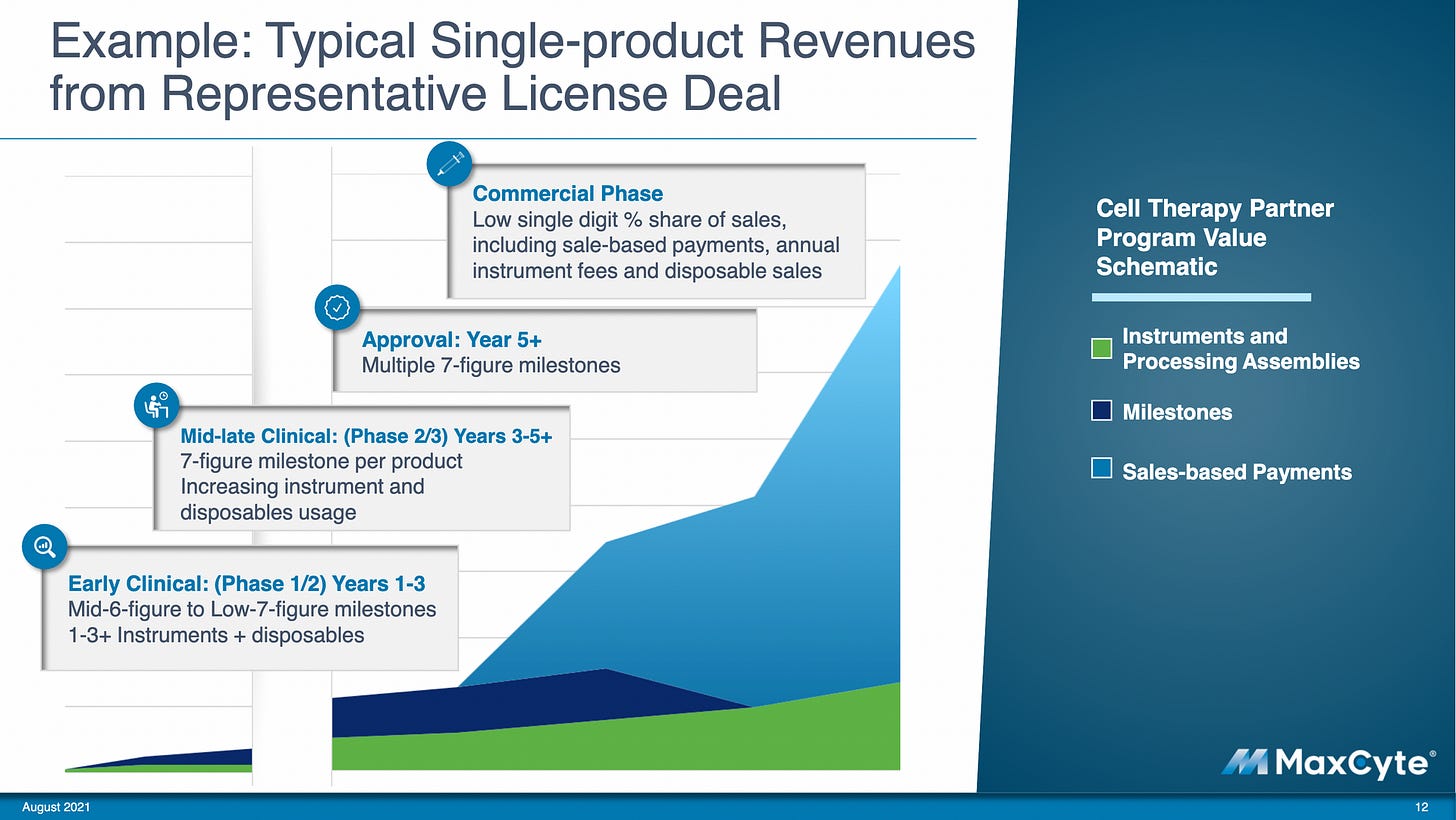
As the gene-editing market grows, the demand for effective transfection technologies like MaxCyte grows. You get the upside of cell therapy market growth while diversifying risk across a dozen, potentially hundreds, of therapies. Here's an example of revenue MaxCyte can receive over time from a single partner company:
MaxCyte's core business is license fees for companies developing cell-based therapies. As a result, their machines are like very expensive K-cup machines that partner companies don't own.
But it doesn't stop there. They don't just license out their machines — they also get milestone payments and royalties.
Milestone payments ensure MaxCyte gets paid even if not all their partner products succeed. They have potential milestone revenues of over $950 million based on deals they've already made, a number that dwarfs their $26.2 million revenue in 2020.
Royalties in the low single digits reward MaxCyte for supporting therapies that have successfully come through the other side of the clinical development funnel. For example, CRISPR Therapeutics' therapy for hemoglobinopathies (like sickle cell disease), CTX001, uses MaxCyte's technology. CTX001 has an excellent chance of getting FDA approved. One estimate thinks MaxCyte can cumulatively gain $427 million over the first 10 years ($354 million from royalties) from this one treatment alone:
MaxCyte is also the Illumina of electroporation companies. If you want to manufacture clinical-level ex vivo gene therapy, you use electroporation to deliver gene editors. If you want to use electroporation, there's a good chance you use MaxCyte.
For ex vivo treatment, all gene-editing companies (except Graphite Bio, which appears to be using a combination of viral vectors and electroporation in some preclinical papers) use electroporation as the method to deliver gene-editing components into the cell. Out of these, 7 of them use MaxCyte. Intellia probably uses MaxCyte, though that hasn't yet been officially announced. For this post, this list only focuses on gene editing companies, though MaxCyte has many other cell-based therapy partners.
Potential risks and unknowns
The biggest risk I see for MaxCyte is that there may be ways to bypass the need for ex vivo treatments entirely. If so, that would mean electroporation as a whole may be used less often for gene editing.
I haven't seen any investors keen on MaxCyte mention this new paper published in June 2021 by Dr. Jennifer Doudna's lab. The paper describes a new approach to engineer viral-like particles to deliver gene editors into T-cells without the need for electroporation, which can be used both ex vivo and in vivo:

This is still just proof of concept, but it could mean treatments like CAR-T would not need to go through the whole process of gene editing and cell expansion ex vivo then infusion into patient's bodies. Instead, targeted gene editing could happen directly in the body through a drug. Doudna has talked about this new delivery method and its implications:
"We've been thinking about [CRISPR-engineered T cells] and wondering if there might be an alternative in the future. Wouldn't it be great if you didn't have to do all of this [ex vivo editing/expansion] and you had a way to provide a one-and-done injection or a pill that can be taken?"
Jokes aside, if these innovative new delivery methods work inside the body, this is great news for future patients because it would reduce cost and increase accessibility to CRISPR treatments. The biggest concern is whether this can be done safely and effectively, without causing off-target editing mayhem.
I'm not sure what this would mean for MaxCyte, but it is an indication that scientists are thinking beyond electroporation. Granted, it will still take a long time for in vivo therapies to be approved and the cost/time delays/regulatory hassle to switch delivery strategies for existing therapies in the clinical pipeline would be substantial, so it’s likely a distant long-term threat. However, while MaxCyte has secured partnerships with pioneering gene-editing companies, it doesn't necessarily guarantee the companies will keep using MaxCyte and that they won't find another workaround in the coming years. ARK seems to think this is the case since they had a slide dedicated to how gene therapies could shift from ex vivo to in vivo editing:
MaxCyte is notably absent in ARK’s Genomic ETF, likely for this very reason.
The big picture
Overall, the value of MaxCyte is under-recognized and is probably a bigger deal than people realize. As long as MaxCyte continues to be the go-to solution for cell transfection ex vivo, we should expect more cell therapies that leverage the company's technology to grow over time. The snowball effect comes as more licenses, partnerships, and commercialized therapies gain momentum.
At the same time, there is still a long-term risk to investing in MaxCyte. Gene therapies could shift away from ex vivo editing entirely. Does that mean electroporation has the potential to become obsolete for gene therapy in the future? Probably not, though there will likely be more competition.
Lastly, I mostly focused on gene editing here, but many other applications rely on electroporation — the development of small molecule drugs, vaccines, and therapies based on protein and antibody expression are all part of the future of the cell-therapy revolution.
Thank you to @saharainvesting for feedback on an earlier draft of this piece. You can follow Health & Wealth’s newly created Twitter account @healthwealthgen for the latest updates.
Over to you — what am I wrong about or what might have I missed? Your feedback helps me make this great.
If you enjoyed this post, subscribe and tell your friends!
Thanks for reading!
Christina










What % of revenue comes from non gene editing applications ?
Christina, there is something "grossly" (pardon the pun) wrong with the table in the MaxCyte story. (1) If, as you say, gross margins are 89%, then the gross profits in the table are way off, and (2) net margins at a constant 3% mean either (a) the business does not scale (I find this unlikely), or (2) someone has scaled "fixed" costs like GS&A, R&D as if they were variable.
I like your work, though! Martin J. Monroe, PhD