Scaling and Expanding Genetic Screening on Embryos
Exploring the future of PGT and what to prioritize
Welcome to Health & Wealth! If you’re new, you can subscribe for free to learn about genomics, life science companies, and my fresh insights on the innovation theme park of biology — delivered directly to your inbox:
I’ve had this piece in mind but haven’t put it all together until now. The world of embryonic genetic screening is filled with acronyms — two that will be mentioned often are preimplantation genetic testing for aneuploidy (PGT-A) and preimplantation genetic testing for monogenic disorders (PGT-M.) As always, feedback is encouraged and welcome.
Article Highlights
Current IVF volumes meet only a small fraction of medical need. The current $25 billion global IVF industry can grow to $400+ billion. As IVF grows precipitously, the need to scale PGT and increase accessibility by reducing cost will be inevitable.
Two approaches that could lower PGT-A costs include combining image-based AI embryo evaluation as a first-line screening tool with PGT-A using low-pass whole genome sequencing.
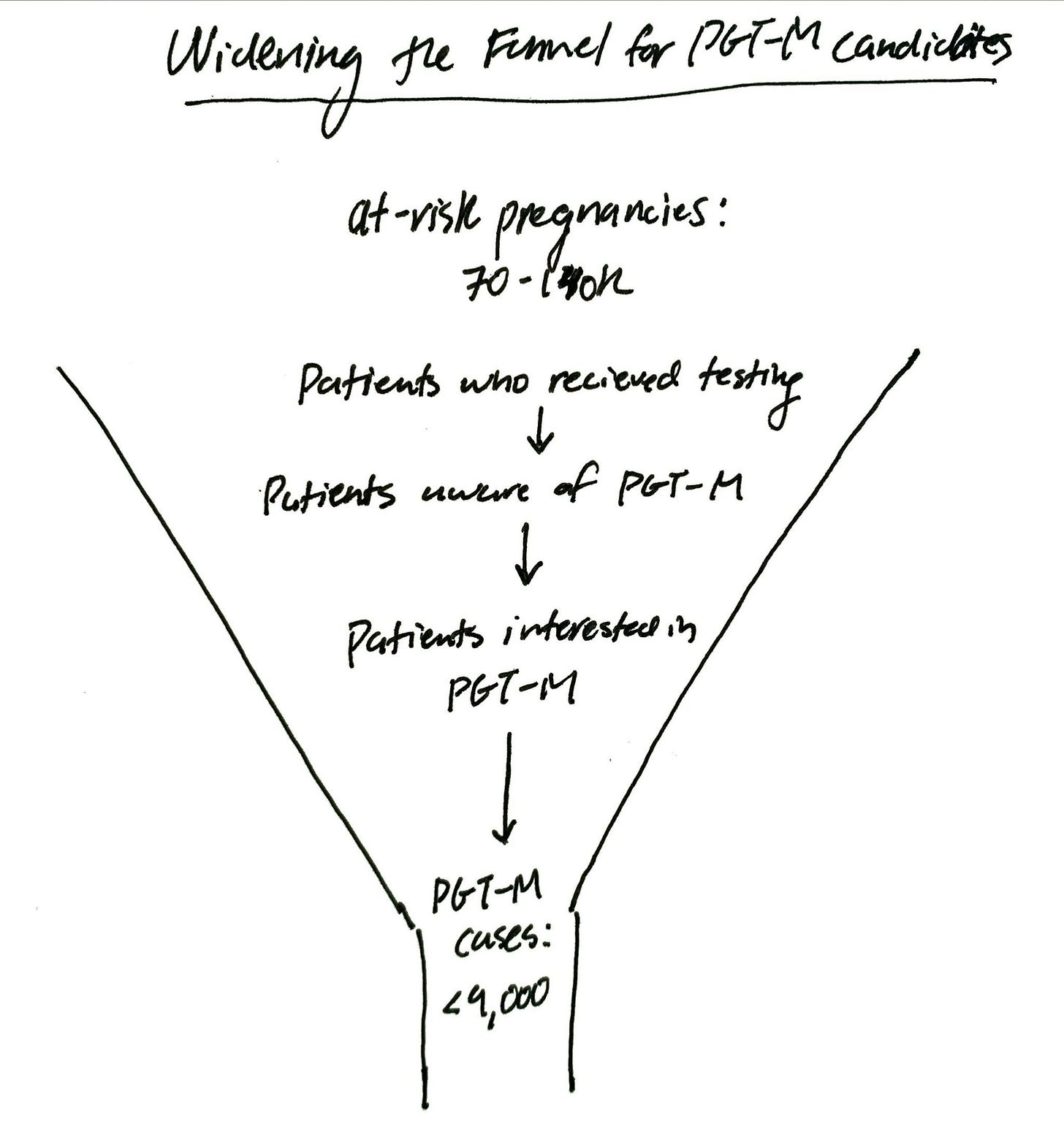
Annually, 70-140k people in the US could need and want PGT-M for genetic disease prevention but are getting lost in the cracks. How do we reach these at-risk pregnancies?
A new technique called primary template-directed amplification (PTA) allows labs to bypass much of the tedious process needed for PGT-M, cutting costs and turnaround time.
Why scaling embryonic genetic screening is inevitable
I’m not the first to say this but I’ll reiterate it: in vitro fertilization (IVF) is vastly underutilized and one of the most attractive business opportunities in healthcare today.
Current IVF volumes meet only a small fraction of medical need. Only 2% of couples struggling with infertility worldwide can access IVF, leaving 98% of patients untreated. If we add other reasons people may seek IVF including recurrent miscarriages, genetic disease prevention, oncofertility preservation, LGBTQ family building — we can see the current $25 billion global IVF industry delivering ~500k babies annually turn into $400+ billion with 20 to 30 million babies a year.

Another way to show how far we have to go to meet the expected demand is in this graph. The US is well below the demand threshold compared to leading countries like Israel, Japan, and Denmark.
Against this backdrop, genetic testing on embryos, otherwise known as preimplantation genetic testing (PGT), accounts for over half of IVF cycles in the US today. While the total number of IVF cycles has increased modestly over the years, the most striking change is the proportion of cycles that used PGT—a 10-fold increase in just nine years.

Although the cost of PGT has been decreasing, it remains expensive and inaccessible to many. Depending on the clinic and lab, it can cost patients between $3-10k per IVF cycle — on top of standard IVF costs.
What if there is a way to:
Reduce PGT to less than $1000 per IVF cycle (not per embryo)?
Eliminate the need for probe development in PGT-M, lowering cost and patient hassle?
If we assume that in the future, half of the 29 million IVF babies worldwide would be genetically tested as embryos at $1k per cycle, that's $14.5B in annual revenue, over half the size of the entire IVF industry today. While this may sound lofty, as IVF grows precipitously, the need to scale PGT and increase accessibility by reducing cost will be inevitable. How can this be done?
Make preimplantation genetic testing (PGT) available for everyone, not just the wealthy
Let’s start by understanding the current PGT landscape. Many PGT labs have historically been owned by private equity or as subsidiaries of commercial genetics labs.
Private equity-owned PGT labs are often not sufficiently incentivized to expand their market size at the expense of driving down prices. While they may be stable businesses, I don’t know if they can scale fast to meet the vast, unmet demand for PGT.
Meanwhile, commercial genetics labs have struggled to maintain sufficient margins for PGT. Invitae stopped offering PGT-A in 2022. Natera started outsourcing PGT testing and, as of November 2023, has stopped accepting samples for PGT-M, a huge loss for patients because many insurance companies only provide coverage for PGT if serviced by Natera. What’s more, when PGT is done, the exorbitant costs are often passed onto the IVF patients without sufficient notice.
Some PGT labs opt to remain cash-pay businesses with minimal contracts, while others have insurance contracts that can increase patient access to PGT through coverage. I’ve previously written about the unit economics of genetic testing labs, highlighting why it’s a difficult line of business. PGT is not immune to these issues, although today IVF exists as an oligopoly and contracts with clinics remain heavily relationship-driven. Clinics can also bill patients for PGT directly, adding to price variability.
$1,000 PGT-A per IVF cycle?
About 90% of PGT performed is PGT-A, which is used to prioritize the transfer of chromosomally normal (euploid) embryos. Two approaches that could lower costs include combining image-based AI embryo evaluation as a first-line screening tool with PGT-A using low-pass whole genome sequencing (WGS).
Image-based AI embryo evaluation can help rule out embryos that are unlikely to lead to a healthy live birth. For embryos with a high euploid likelihood score, patients can opt to directly transfer the embryo without needing to freeze it, resulting in less friction, money, and medications. For embryos on the borderline, biopsy for PGT-A can be used as confirmatory testing to help prioritize remaining potentially viable embryos. Retrospective studies show that ~80% of the high euploid likelihood calls through imaging were concordant with PGT-A results. In the near-term future, this can be a cost-effective triage tool to determine if PGT-A is necessary and if so, which embryos to test.
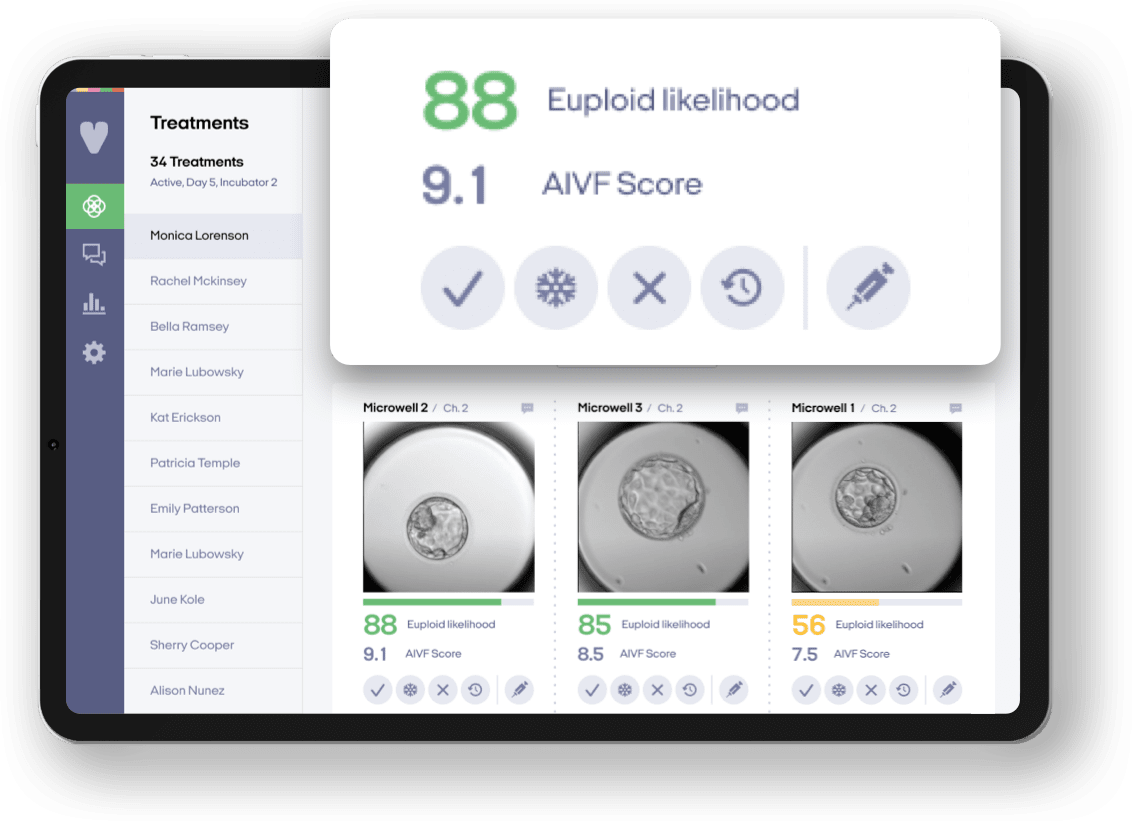
One way to lower the cost of PGT-A could be to use low-pass WGS. Instead of reading every piece of DNA many times, low-pass WGS reads the DNA fewer times — just enough to get a general overview. At 0.4-5x coverage (the number of times the DNA is read), it’s sufficient for detecting chromosomal abnormalities in embryos (whole/segmental aneuploidies and mosaics.) For large-scale processing, costs can drop to ~$50 per embryo. Some PGT-A labs may already be using this, though I’m unsure of the cost.
While a comparatively “barebones” approach, price and accessibility become more pertinent when considering scaling PGT to millions of cycles a year.
Another critical cost component is from the embryology lab before samples are sent to the PGT lab. Clinics can charge $400-500 per embryo to biopsy and freeze embryos (depending on region). This accounts for a significant portion of patients' PGT-A costs, which can be addressed by eventually automating the biopsy procedure.
Now let’s look at expanding access to genetic disease prevention.
Expand access to preimplantation genetic testing for monogenic diseases (PGT-M) to people who need and want it
Today, we can prevent rare genetic disorders for the next generation. Couples with a known risk of passing down a single gene disorder can genetically test their embryos created in the lab and choose to transfer an unaffected embryo. This can be a game-changer for families who wish to prevent undue disease burden for their children such as sickle cell disease or cystic fibrosis. This is known as PGT for monogenic diseases (PGT-M).
How many potential PGT-M cases are there in the US? Some napkin math:
WHO estimates about 1% of the population is affected by a monogenic condition (higher if include adult-onset, less severe conditions)
~3.5 million babies born in the US annually means over 35k babies are born with a monogenic condition
Depending on the inheritance pattern, that’s ~70-140k at-risk pregnancies annually
How many PGT-M cases are being performed today?
PGT-M makes up less than ~5% of all PGT cases
~180k IVF cycles included PGT in 2021 means less than 9,000 PGT-M cases annually
There are a few PGT-M labs in the US: Igenomix, Cooper, Juno, RGI, Orchid, Sequence46, and Luminary. One of the larger labs, CooperSurgical, reports 1,000 PGT-M cases a year and a total of ~8,000 cases over 8 years. If other lab volumes are similar or lower, <9,000 per year seems reasonable.
How do we reach the 70-140k people annually who could need and want PGT-M for genetic disease prevention but are getting lost in the cracks? Instead of waiting for patients to come into the fertility clinic at the bottom of the funnel already knowing they want to do PGT-M, how do we widen the top of the funnel?
This is where the tribal knowledge of genetic counselors, marketing and sales executives, and patient advocates comes in. Pediatric genetics clinics understand parents’ journeys to having a child with a rare genetic disorder. Individuals and couples at reproductive genetic risk can tell you how they got there and what testing options they were offered and pursued. Genetic testing labs can share various strategies for expanding the total addressable market for genetic testing. This extends beyond expanded carrier screening to include adult-onset dominant conditions.
Of course, there is still a long way to go before IVF is truly accessible and cost-effective. However, with automation and other innovations, it’s possible to get the price down to about $15,000 to have an IVF baby. I’ll focus on addressing some of the downstream challenges of PGT-M.
Addressing technical challenges of PGT-M
PGT-M remains both tedious and costly. Each case requires developing custom probes for linkage analysis, a process tailored to the patients’ genetic background.
Highly individualized — unique test customized for each family, requiring genetic testing of the couple and ideally both sets of grandparents for the specific genetic variant
Labor-intensive — takes a PGT lab genetic counselor on average 5-6 hours per PGT-M case
Time-intensive — 2-3 months turnaround time, 4-6 weeks to develop probes before embryo biopsy can be received, more if patients can’t coordinate testing for extended family members
Most of the cost for PGT-M today comes from developing the probes. The average base price starts at $3,000 plus $400 per embryo. That doesn’t include the cost of testing adult family relatives to create the probes.
These technical challenges will likely soon be overcome with methods that offer comprehensive PGT. One particularly promising technique is a new way of copying DNA from small samples (5-10 cells for embryo biopsies) called primary template-directed amplification (PTA). The gist is the amplification happens directly from the original genome, reducing bias and errors. It’s like a photocopier that only copies from the original document instead of random copies of copied documents.
PTA is just entering clinical use. BioSkryb is the company commercializing PTA, and CooperSurgical is one lab that has a deal to distribute its PGT kits. PTA is still undergoing validation and has important clinical caveats today, but it is worth noting. By enabling accurate detection of genetic variants directly from the embryo, it eliminates the need to test family members. This simplifies workflows while maintaining accuracy comparable to current industry standards like linkage analysis. What’s more, PGT-A and PGT-M can be combined in one workflow with eventual turnaround times equivalent to PGT-A timelines, around 7-10 days instead of months. I don’t know the conversion rate from “aware of PGT-M” to “pursuing PGT-M,” but I imagine this could help widen the bottom half of the funnel.
Future horizons in PGT
Lastly, I would be remiss if I didn’t mention PGT using high coverage WGS (PGT-WGS). PGT-WGS represents a glimpse into the future, pushing the boundaries of what genetic testing can offer, including many inherited and de novo gene mutations, polygenic risk scores, and rare structural variations. However, its cost and complexity currently make it less practical for large-scale adoption. There are lower-hanging fruits, like improving access to PGT-M to make genetic disease prevention a standard, not a privilege.
The immediate goal should be to scale and expand access to embryonic genetic screening for the future health of babies born through IVF in a responsible and beneficial way. It’s better to be agnostic to methodology but sensitive to patient benefit, cost, and turnaround time. Offering the “most advanced” embryo testing at a high cost may not be the most viable option for most.
But like other IVF services, there can be the Ritz-Carlton of PGT and the Holiday Express versions. I believe in both reproductive choice and equitable access to testing. In the long term, lower costs of PGT-WGS may make it a viable option for routine use. Until then, the focus should remain on practical, cost-effective solutions that can serve the needs of a rapidly growing IVF population without sacrificing quality or accessibility.
What am I wrong about or what might I have missed? I write to learn and sharpen my thinking, but also to invite others to correct my blind spots. I'd love to chat with you if you have ideas about increasing access to PGT-M. I’m also interested in breaking down the cost structure of PGT — any leads here would be much appreciated.
Thank you to Andria Besser, Amber Kaplun, Lauren Moissiy, Rawan Awwad and others for feedback on an earlier draft of this piece. My sincere gratitude to those willing to lend their time and expertise.
Consider sharing this piece with your network and reach out with questions or feedback.
Some chatGPT humor:
Stay curious,
Christina








This was such an insightful read. Would love to have you speak more on this on a panel! Sent you a LinkedIn message :)