Twist Bioscience: DNA synthesis as a service or product?
Part 2 — Exploring Twist Bioscience’s competition and defensibility
Hi there! Welcome to Health & Wealth — It’s been a minute, hope you’ve all been well. After publishing my overview thesis on Twist Bioscience, I received some questions from folks about competition and the defensibility of DNA synthesis as a business. It’s worthwhile to understand; here are some of my thoughts. If you are new, you can join here — feedback is encouraged and welcome.

In case you missed it, take a look at the piece I wrote on Twist Bioscience TWST 0.00%↑ for more context:
Twist Bioscience: Silent partner in the new era of biology
(estimated reading time: 10 mins)
Here I talked about how there are a few other embedded moats apart from scale, enabling Twist to improve margins as revenue ramps up. In diagnostics, emerging diagnostics such as liquid biopsy for cancer detection have customer stickiness because the components of tests are set after receiving FDA clearance. After that, switching costs for sequencing sample prep and DNA probes are high.
In therapeutics, creating customized tools for their biopharma customers improves Twist’s libraries over time, which can be productized and sold to other customers. There exist some network effects as the product becomes more valuable as more customers use Twist. This gives them pricing power and the ability to share value creation through milestones and royalties.
More broadly speaking, exponential drops in synthesis cost introduce challenging market dynamics because more volume is needed to make the same topline revenue. This challenge pushes many life science tools companies to diversify their business models, as evidenced by Illumina’s recent announcement that they too will get into drug discovery:


This gets us to the question: should DNA synthesis be sold as a service or product?
Today, Twist’s business model straddles both, starting as a service-only DNA synthesis company and later productizing their drug discovery capabilities to antibody libraries and now high-throughput antibody production (delivering the end product proteins themselves).
Instead of making synthetic DNA a substitutable commodity, Twist sells complements — products bought are bundled with other ones (i.e., large-scale DNA synthesis to power protein drug discovery). As Joshua Elkington describes it:
“A great way to get ahead is to focus on complements - they are non-competitive most of the time and can build powerful moats that are historically insurmountable by others. Ultimately, the basic strategy with this framework is that demand for a product increases when its complements’ price decreases.”
But there are other ways to slice and dice. Let’s further break down the competing technologies and business models. Design considerations for DNA writing platforms to optimize for are:
decrease turnaround time
decrease cost
increase yield
increase accuracy
And there are two competitive fronts Twist Bioscience faces:
Existing large commercial competitors
Emerging technologies using enzymatic synthesis
Existing large commercial competitors
Companies like IDT, GenScript, and Agilent existed before Twist’s founding in 2013. They offer DNA synthesis through chemical synthesis, NGS sample preparation, and other related tools shipped to customers.
Currently, Twist Bioscience is the frontrunner in developing commercially competitive synthesis technology. They win out on price, volume, speed, and accuracy. The one limitation with Twist’s silicon chip synthesis platform is sequence length — with a maximum gene fragment length of 1,800 base pairs compared to longer gene fragments created through traditional chemical synthesis. Twist also has a gene calculator that compares cost savings to commercial competitors depending on sequence length and the number of genes ordered.

Today, commercial DNA synthesis is primarily a service model dependent on centralized factories — companies make the DNA requested and ship it to their customers. But what happens if people want to make DNA in their own labs? That’s where enzymatic synthesis can come in.
Emerging technologies using enzymatic synthesis
More recently, several startups have been working to improve the synthesis process by using enzymes, instead of chemical reactions, to make DNA. This process would mimic nature’s ability to efficiently replicate entire genomes. In theory, once cracked, the approach can generate longer molecules than chemical synthesis can in a shorter timeframe.
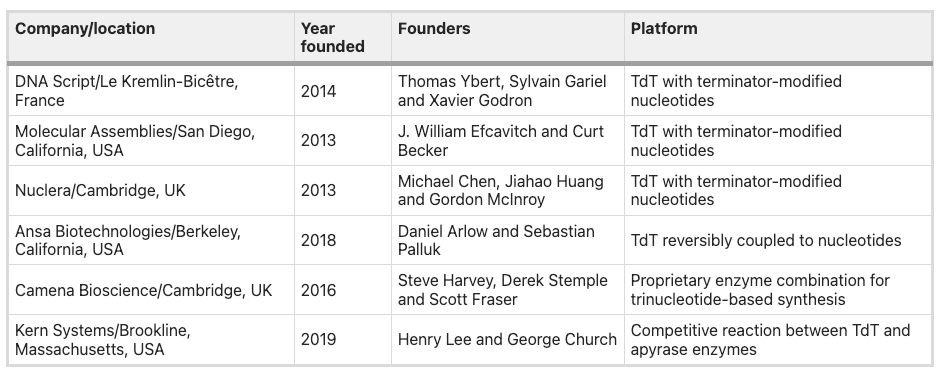
However, enzymatic synthesis is still in the earliest commercialization stage and is currently much more expensive than existing methods. DNAScript launched its benchtop DNA printer in June 2021, which offers much lower throughput (96 oligos per run, ~two runs per 24 hours) and shorter sequence lengths (up to ~80 base pairs). You can see the process in action in this video. In short, not yet super practical for commercial applications or gene synthesis.

Despite current-day limitations, enzymatic synthesis offers the future promise of a distributed model — one where customers can buy synthesis machines (as they do with sequencing machines) to make DNA in their own labs. This follows the Illumina-like “razor and blade” model in which customers have their own machines and order reagents and consumables. By contrast, Twist’s chip production process today is too hard to set up outside a specialized facility.
Where would enzymatic synthesis be useful?
In the future, centralized high-throughput synthesis will be complemented by distributed local synthesis. I see three areas where enzymatic synthesis will be required that Twist can’t currently capture:
Small-scale research applications that want same-day turnaround times. These are users within academia or emerging biopharmaceutical companies who only need a few genes at a time. They value being able to print DNA on demand instead of waiting several days to receive DNA by mail. DNA Script’s CEO Thomas Ybert sees enzymatic synthesis as an essential tool to accelerate research:
“The ability to print oligos in virtually any lab could have a tremendous ripple effect on research workflows. If a lab can write its own genetic material within 24 hours, it no longer needs to plan workflows around custom oligo deliveries. This could profoundly accelerate the iterative development process for research tools, diagnostics, vaccines and therapeutic antibodies.”Companies want in-house archival DNA storage for sensitive data. Archiving data in DNA can be a compact, durable solution to meet the demand of exponential data storage needs. But some companies may not want to send their data to Twist’s facilities to manufacture DNA storage chips for them. In that situation, enzymatic synthesis is needed for distributed digital information storage. Companies would have their own synthesis machines for in-house DNA storage (and later data retrieval).
Ultra-long DNA synthesis to write entire chromosomes or genomes. This isn’t so much about a distributed model as addressing the technical limits of chemical synthesis to write super long and complex DNA. Ansa Biotechnologies, an enzymatic synthesis startup, believes this to be the critical bottleneck. They’re forgoing the printer route (DNA Script’s approach) and directly going after the centralized service model Twist offers. Interesting to see divergent business models even amongst enzymatic synthesis startups. I don’t have sufficient information to comment on the near-term viability of Ansa’s approach. Still, their CEO Daniel Lin-Arlow says centralized printing is the best way to control quality:
“The customer wants exactly the sequence that they want, and if we centralize the production of that, we can QC [quality control] the DNA before we send it out the door and make sure that we’re sending them exactly what they want. If they have the printer, the burden of QC is on them, and they don’t want to fuss with this stuff.”
What has Twist’s response been to emerging third-generation technologies?
On a high level, Twist Bioscience is focused on delivering what’s commercially ready and viable (their silicon microchip, a “second-generation” platform). In parallel, Twist also announced in January 2022 that they’re developing its own enzymatic synthesis tools. As the third-generation platform improves, Twist will begin deploying capital to offer enzymatic synthesis for specific applications:
“Today, we use phosphoramidite chemistry in our robust commercial infrastructure to deliver billions of bases in DNA and RNA products to serve almost 3,000 customers in fiscal 2021 alone. We will continue to use this proven chemistry as we optimize our enzymatic capabilities. We see enzymatic synthesis playing a critical role in applications like DNA data storage, primers and probes for PCR, cell-free production of plasmid DNA, qPCR assays, decentralized DNA synthesis, and look forward to expanding our markets by offering these applications either directly through our channel or as OEM to our customers in the future.”
They haven’t released many details about their enzymatic synthesis capabilities, except that they’ve been tinkering to improve the error rate, length of DNA, speed of reaction steps, and perhaps most importantly, overall cost. A couple of key parameters to solve for:
Reduce the cost of reagents, specifically by lowering the concentration of nucleotide triphosphate, an expensive reagent needed for each base pair to be added.
Optimize the TdT enzyme used by screening hundreds of thousands of different enzyme variants to find the “chosen one” that can deliver the best reaction times without sacrificing accuracy.
The bottom line
Twist’s approach to continue growing its core commercially viable business while preparing for the next innovation wave is pragmatic and adaptive. There will always be demand for high-throughput, low-cost synthesis. The competitive pressure of enzymatic synthesis hasn’t yet arrived because it’s far from cost parity to chemical synthesis, but Twist seems equipped to evolve its offerings when the transition to third-generation platforms proves to be scalable and commercially viable in the coming years.
The centralized lab-as-a-service model is dominant today. In the future, benchtop synthesis machines will inevitably give rise to do-it-yourself synthesis for applications that require ultra-fast turnaround times. We will also likely see the technical ceiling of DNA synthesis being raised, like entire genomes being synthesized from scratch. Finally, the cohort of enzymatic synthesis startups adds some competitive heat, though there doesn’t appear to be a clear frontrunner in these early days.
What am I wrong about or what might I have missed? Your feedback helps me make this great. If you’re new, you can subscribe to have my future writings delivered to your inbox:
My publishing cadence has slowed as I’ve been dealing with a severe bout of insomnia these past few weeks. It would be disingenuous of me not to “eat my own cooking” by not focusing on health first. Thanks for your patience and continued interest!
Christina