A look under Ginkgo Bioworks' hood — Understanding their science and business
Part 2 — Taking a closer look at Ginkgo's biofoundry, specific products they've designed, and the value of a horizontal model
Hi friends 👋
Welcome to Health & Wealth — your weekly source of the latest health research and biotech trends. Several people have commented or messaged me telling me that they appreciated how balanced my first piece on Ginkgo was. This remains my intention to give you honest analysis and commentary on relevant topics. Thank you for being part of this corner of the internet ✨
If you are new, you can join here and share within your network — word-of-mouth is how I grow. Feedback is encouraged and welcome. Happy reading!
Article Highlights
The jury on whether Ginkgo DNA 0.00%↑ will succeed or fail is still out. To understand the company, you need to understand the purpose of biofoundries and what they've achieved with their state-of-the-art biological factories to date.
The most potentially lucrative and impactful product Ginkgo has been involved with is working out a way to boost production yields of vaccinia capping enzymes (VCE) for Aldevron. VCEs are needed to make mRNA therapies (including Moderna's COVID vaccine.)
Ginkgo has designed prototypes of microbial therapeutics for Synlogic, but none of their engineered strains have made it to clinical trials yet.
Scaling manufacturing is not trivial. Going from bench-scale reactors to industrial fermentors introduces many variables that can be difficult to model or predict.
As the debate saga on Ginkgo Bioworks (DNA) and the future of synthetic biology continues, two critical questions remain:
Does their science show real potential, and are the use cases compelling enough?
Does their business model add up, and is it sustainable?
In case you missed it, I recommend you read part 1 of my series on Ginkgo for some context priming.
To begin answering those two questions posed, we need to look at the following:
How Ginkgo's biofoundries work
Case studies on Ginkgo's customers
Technological and scaling challenges
Business risks and unknowns
Key catalysts to watch for
I will preface by saying I'm not inside Ginkgo. Anything I say is researched through my deduction. I rarely am severely critical of a person or company unless they're doing something egregious or fraudulent. I've worked at a controversial startup and know firsthand how disheartening and traumatic it is to see outsiders bashing on what I've worked so hard to contribute to building. I will not do the same for others.
I don't pull any punches, but I will call it as I see it. Of course, I may be wrong, in which case I appreciate it when people tell me how I'm wrong.
With that — let's get into it.
How Ginkgo's biofoundries work
At the heart of Ginkgo is their "biofoundry" — a nod to the semiconductor industry's foundries for making chips at scale. But what is a biofoundry?
Biofoundries are essentially hyper-specialized, mostly automated factories that help discover, engineer, and manufacture organisms. However, unless you already have deep expertise in cell engineering, it can feel like a black box — no one really knows everything that goes inside of it. It's part of the reason why it's hard to understand Ginkgo fully.
High-level theory: Directed evolution in the lab
The high-level goal of Ginkgo's foundries is to identify molecules with commercial value for their customers and reprogram an organism with the most optimal, desired qualities.
What does that mean exactly? Let's say you're trying to get from point 1 (unmodified strain) to point 2 (global maximum of this strain's desired function):
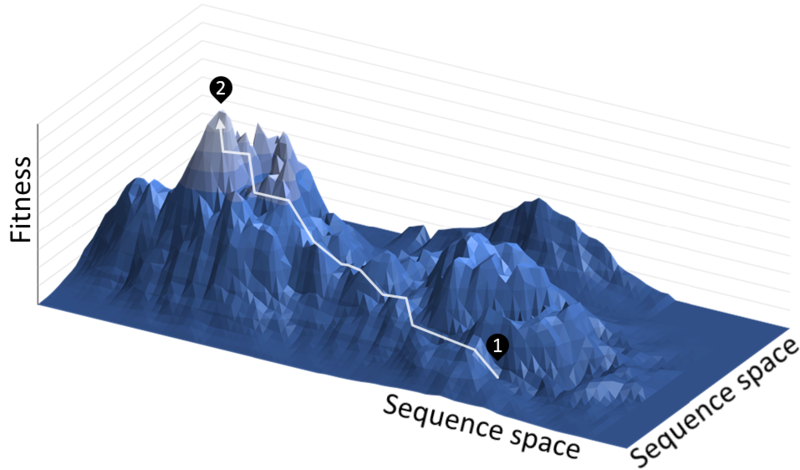
There are many ways to traverse the terrain from point 1. Without a predetermined map, it can be hard to tell which way to go. You can also fool yourself by thinking you've improved the strain, but you've only ended up at a local — not global — maximum.
Therefore, in the initial design stages, the way forward is to test many different versions of genetically modified cells and select the ones demonstrating the most potential to continue experimenting.
Dare I say it and make the analogy — like Squid Game but for cells? 🙈
But this approach is only feasible if you can support high-volume, rapid experiments. The first generation of synthetic biology was artisanal and couldn't support this "roll the dice 100,000+ times" method. This is where modern-day foundries come in.
Building more automation and a catalog of biological parts
I've seen some criticize Ginkgo for having nothing but an army of PCR machines. Others have questioned Ginkgo's role in creating organisms, given how easy CRISPR makes it to edit DNA.
The truth is these arguments overlook just how hard it is to write biology. We can borrow life's tools that already exist to tweak certain aspects, but building brand new organisms remains a major overhaul.
Others have already described the foundry process (see Axial, ARK, and Ginkgo posts). However, I primarily want to highlight two key points:
Ginkgo frequently refers to their growing "codebase" as a way for them to iterate faster and reduce costs as they scale. What does this mean?
Their codebase is essentially their catalog of parts — the biological equivalent to design specs for IKEA furniture parts — all the hardware, wood frames, etc., needed to build furniture. Sometimes, these parts can be reused to assemble different furniture pieces. Similarly, as Ginkgo expands its codebase, more biological parts become reusable across many different programs.
Ginkgo frames this iterative learning as a way to win in the long run, even if specific programs fail:
"Ginkgo's made the choice that we would rather have broad exposure to the entire industry, and we win with the winners, and when products on our platform fail to get to market, because the clinical trials fail to work, so the science doesn't work, no problem. We generated interesting reusable learnings, and we'll dedicate that capacity to the next thing. We do not live or die on any given program."
— Anne Wagner, Ginkgo's SVP of Corporate Development, at Wells Fargo Healthcare Conference 2021
I might talk later about how this mentality is risky if taken to the extreme, but for now, let's take it at face value.
While Ginkgo has yet to show they can scale their customer base, they have demonstrated increased foundry output while decreasing the cost per strain test over the past few years.
I hesitate to call this a win quite yet because having the most state-of-the-art biofoundry will amount to nothing if their customers don't love what Ginkgo builds for them. But this is still encouraging and a necessary prerequisite to enable exponential improvement to engineering organisms.
Case studies on Ginkgo's customers
Ginkgo talks a lot about how they can address the world's biggest problems by growing anything under the sun:
But in my last post, I mentioned how I wanted to better understand specific case examples of what they've done and what their customers have decided to do with their engineered organisms after the handoff. It's quite hard to keep track, given many of Ginkgo's partners are themselves early-stage companies, and there is little information available beyond press releases. Here's a table of Ginkgo's partners which seems relatively accurate and up to date.
I will give color to three of Ginkgo's customers — Aldevron, Synlogic, and Joyn Bio.
Aldevron — making capping enzymes for mRNA vaccines
The most potentially lucrative and impactful product Ginkgo has been involved with is working out a way to boost production yields of vaccinia capping enzymes (VCE).
VCEs are a critical component in mRNA therapies and vaccines because they help the body recognize foreign mRNA. Without this capping enzyme, the introduced mRNA is seen as foreign and vulnerable to degradation before it successfully translates its instructions into functional proteins.
Within a year, Ginkgo helped Aldevron (recently acquired by Danaher) increase production yields of VCE by 10x. Notably, Moderna's COVID-19 vaccine utilizes VCE from Aldevron. Aldevron is given exclusive rights to the protocol conditions, and in exchange, Ginkgo is expected to collect royalties from this project beginning at the end of 2021.
Aldevron is not the only company supplying capping enzymes, but mRNA therapies extend beyond COVID applications. Thus, many other opportunities can be captured in the future.
The bottom line: VCE is an example of a known molecule, but Ginkgo figured out a way to produce it at unprecedented scales to meet the sudden demand of billions of vaccine doses. This appears to be a clean deal (Aldevron isn’t part of Ginkgo's intricate web of subsidiaries), and I look forward to seeing concrete revenue numbers materialize soon.
Synlogic — designing microbes to treat metabolic diseases
Synlogic focuses on creating "living medicines" — specialized probiotics to help treat severe genetic diseases like Phenylketonuria (PKU).
The standard of care for rare metabolic diseases is often dietary protein restriction — avoid the amino acids that the patients can't properly metabolize. Unfortunately, the consequences of not adhering to diet restrictions are severe — the buildup of specific amino acids like phenylalanine can cause neurological disorders. Synlogic asks whether it's possible to engineer strains that make enzymes to break down these amino acids. If successful, patients can orally take these microbes to nullify the risks of toxic amino acid buildup. Functionally, it's equivalent to insulin shots for people with Type 1 Diabetes who don't produce insulin in their bodies.
It's a worthwhile and venerable goal — now let's look at what Ginkgo has been able to do for Synlogic. I'll present the promising news here first and then describe where things get a bit murky.
Pre-clinical data for branched-chain amino acid-related metabolic diseases treatment
A pre-print article from March 2021 describes Ginkgo's work in developing strains for Synlogic to lower branched-chain amino acids (BCAA) such as leucine in healthy non-human primates. This prototype suggests a viable approach to treating rare metabolic genetic diseases such as maple syrup urine disease (MSUD).
Phase 2 data for Phenylketonuria (PKU) treatment
Synlogic's most mature product is an organism that breaks down phenylalanine (Phe) for PKU patients. They recently announced positive phase 2 data in which an engineered E. coli strain effectively broke down Phe in the GI treat of patients with PKU. They're now looking to see the microbe can decrease Phe levels in the blood.
However, the strain used in these clinical trials is not the one Ginkgo initially made for them. Instead, Synlogic ended up going with strains designed by a combination of in-house capabilities and a collaboration with enEvolv (a startup acquired by Zymergen). This leads me to question whether Ginkgo's platform wasn't quite up to the task:
"The particular organisms Ginkgo helped engineer were not successful after they failed to 'reach our criteria to advance the project' into clinical testing, Brennan [CEO of Synlogic] says. Instead, this summer Synlogic announced it would begin human tests of a new version of its E. coli engineered by enEvolv, a startup recently purchased by Zymergen, which she says brought capabilities to the project that Ginkgo did not have at the time." [MIT Tech Review article]
In this particular case, Ginkgo still owns ~30% of Synlogic, so it doesn't really matter which strain Synlogic went with. But if Synlogic weren't already a related party, Ginkgo effectively would have "lost" this bid.
The bottom line: Ginkgo shows some promise in designing prototypes for microbial therapeutics, but none have made it to clinical trials yet. Synlogic's choice to not use Ginkgo's designed organism for their most mature product adds a big question mark.
Joyn Bio — creating nitrogen-fixing microbes to replace chemical fertilizers
Joyn aims to increase food production by replacing chemical fertilizers with microbes that can fix nitrogen. Joyn is a direct spinout company — Ginkgo has ~35% equity ownership of Joyn, a joint venture with Bayer (BAYRY).
I haven't seen any recent major news from Joyn about commercialization milestones. I mainly wanted to highlight excerpts from this interview I found between Joyn's Head of Bioprocessing Kelly Smith and Culture Biosciences, a cloud fermentation lab that Joyn uses:
"There are two major challenges with biomanufacturing for agriculture. The first one is that it's still a relatively new idea. Large ag companies have a strong history of producing chemicals for pest control. There are still questions about whether a biological product will work, whether growers will buy it, or whether customers accept the fact that we're putting bacteria on their crops. The other challenge is the cost. Ag products have to be inexpensively manufactured for them to be competitive, especially against some of the older chemical products that are generic and cheap at this point." [Kelly Smith, Joyn's Head of Bioprocessing]
Notice that the challenges she cited were primarily about manufacturing their product at scale and a price point comparable to cheap incumbent chemical products. She didn't mention challenges related to identifying or engineering the organism — the two things within Ginkgo's wheelhouse.
She expands more on the specifications needed for their product to succeed:
"We have to look for microbes that grow fast. When you're doing any kind of fermentation-based manufacturing, the biggest cost driver is how long the main fermentation takes. That's where your labor cost comes in, your energy cost, and to some extent your raw materials cost. We try to optimize the fermentation process so that the fermentation can run as quickly as possible. The other big factor is how much it needs to be processed at the end of the fermentation. After you harvest it, every additional step (e.g. handling, formulating) adds costs to the final product, so you want to minimize the number of downstream processing steps that your product needs.
Besides looking for fast-growing microbes, you should also be looking to reduce the cost of your media. Even yeast extract gets to be a bit of a cost problem at scale. You want to work with microbes that can be adapted to grow in a cheap type of broth. Look for inexpensive sources of sugar or choose a manufacturing site where an inexpensive source of sugar is already available. Also, select for microbes that already have the properties you want. It's a lot more expensive to try to adapt the microbe and make it do the things you want in the fermenter than to select for one that has most of those properties naturally."
Scale-up is not trivial. While Ginkgo has shared one example in which fermentation volume of 250 mL was effectively translated into pilot and commercial scale, it remains to be seen whether this will always be the case for future products Ginkgo designs.
The bottom line: Going from bench-scale glass reactors to a steel fermenter (and possibly an even larger steel fermenter) introduces many variables that can be difficult to model or predict. One of the key bottlenecks in the synbio industry will be to move from industrial design to scale-up. Ginkgo needs to design for a broad spectrum of scales — from small batches of specialty fragrance chemicals to commodity products like industrial biomaterials.
Should Ginkgo make their own products too?
I got some DMs after I published part 1 asking me if it's defensible that Ginkgo doesn't make products themselves.
It's an interesting question and something worth pondering — is synthetic biology truly ready for a horizontal model, or is vertical integration the way to go now? Here are some of my thoughts:
Maybe they did try making products before but found it doesn't work for various reasons. It's indeed tough to have breadth while also owning product development from start to finish.
You also need to make product-specific investments — if you want to make a pharmaceutical, you need clinical trial and regulatory expertise. If you're going to make a consumer food product, you need the proper marketing distribution channels. Many first-generation synbio companies died because they went all in and bet on the wrong horse (ex. tackling the oil industry with biofuels).
How do you know if the products you make are the right bets? Zymergen has recently shown us that it continues to be hard, and the potential for failure is high.
The decision not to work on products themselves also seems to be an ideological choice. Ginkgo's CEO, Jason Kelly, has stated that "I am not a product company, and I have no desire to be a product company. People in biotech are brainwashed to think only products matter."
This feels equivalent to 23andMe insisting on being a fully direct-to-consumer company based on principle, which means they have to get FDA approval for each of their medically relevant reports.
Some of their spinoff ventures, such as Motif, essentially act as Ginkgo's side product. Motif shares the same building as Ginkgo, like in-laws crashing at your house. Ginkgo sees the future of synthetic biology as an ecosystem, with Ginkgo at the center of it.
Some closing thoughts
I can't say with any confidence whether Ginkgo will succeed or fail. I respect the founding team and wish the best for them — but driving a ship based on a grand vision with a bent towards having early-stage startups (statistically prone to fail) as their customers is dangerous territory. Wanting something to be true is not the same thing as it being true.
There is more I planned on discussing here, but this post is already quite long. I may extend this series to part 3 soon if there's sufficient interest and I have the mental bandwidth. If you're new, subscribe, so you don't miss it:
I try to keep up a weekly publishing cadence, but carving out time to research and write these long in-depth pieces takes discipline and time — things that I don't always have 🙃 If you have any feedback or want to share any good finds on Ginkgo, let me know on Twitter @healthwealthgen.
I would also love it if you shared Health & Wealth within your network.
Thanks for reading!
Christina










Thank you Christina, I would appreciate a part 3 on Ginkgo. I support your cautious assessment of ginkgo
Any clue about Ginkgo's use of AI in their cellular squid games? I recently read AI 2041, which discussed the current and future prevalence of AI in medicine creation.